MIRA Pharmaceuticals’ stock soared by 318% on Monday, July 22, 2024, following the announcement of positive pre-clinical trial results for their new oral ketamine analog, Ketamir-2. The drug shows promise for treating neurological and neuropsychiatric disorders such as depression, treatment-resistant depression (TRD), and post-traumatic stress disorder (PTSD). The company is considering filing an Investigational New Drug (IND) application to start human clinical trials, bolstering investor confidence and increasing the stock’s trading volume and price.
Key findings from the pre-clinical trials, conducted with Frontage Laboratories and Pharmaseed Ltd., indicate Ketamir-2’s higher efficacy and better safety profile compared to traditional oral ketamine. Notably, it can be administered at higher doses without significant side effects. Behavioral tests demonstrated Ketamir-2’s anxiolytic and antidepressant effects, while its pharmacological profile showed favorable stability and metabolism. Designed for oral use, Ketamir-2 also promises better patient compliance and ease of administration. Future plans include filing the IND application by the end of 2024 and further studies on PTSD and neuropathic pain.
Promising Pre-Clinical Trial Results
The encouraging data from the pre-clinical study has prompted MIRA Pharmaceuticals to consider filing an Investigational New Drug (IND) application. If approved, this would allow the company to commence clinical trials in human patients, a prospect that has significantly bolstered investor confidence, leading to a substantial increase in the stock’s trading volume and price.
Collaboration and Key Findings
The pre-clinical trial for Ketamir-2 was conducted in collaboration with Frontage Laboratories and Pharmaseed Ltd. The results demonstrate the drug’s potential in treating various neurological and neuropsychiatric disorders, including TRD, major depressive disorder with suicidal ideation (MDSI), and PTSD.
Efficacy and Safety Profile
Ketamir-2 has shown higher efficacy and a better safety profile compared to traditional oral ketamine. The trial revealed that it was possible to administer tenfold higher doses of Ketamir-2 without inducing major sedative and other side effects observed with ketamine at lower doses.
Behavioral Tests
Open Field Test (OFT)
Mice treated with Ketamir-2 exhibited significantly greater locomotor activity, higher travel speed, and a trend towards increased time spent in the center of the arena, indicating reduced anxiety levels.
Elevated Plus Maze (EPM)
Ketamir-2 demonstrated anxiolytic effects, with mice spending more time in the open arms compared to those treated with traditional ketamine, suggesting reduced anxiety.
Forced Swim Test (FST)
Ketamir-2 showed significant anti-depressive effects, as evidenced by reduced immobility time in mice, indicative of antidepressant-like activity.
Pharmacological Profile
Ketamir-2’s stability in the bloodstream, metabolism, and protein binding were evaluated, yielding favorable results that support its potential safety and efficacy for human use.
Administration and Compliance
Unlike traditional ketamine, which requires intravenous or intranasal administration, Ketamir-2 is designed for oral use, promising better patient compliance and ease of administration. Additionally, Ketamir-2 is not classified as a controlled substance under DEA rules, potentially simplifying its regulatory pathway.
Future Directions
MIRA Pharmaceuticals aims to file an Investigational New Drug (IND) application with the U.S. Food and Drug Administration (FDA) by the end of 2024. If accepted, this would pave the way for human clinical trials to commence.
Ongoing Studies
The company is conducting further studies to evaluate Ketamir-2’s efficacy in treating PTSD and neuropathic pain, along with additional safety studies. These ongoing investigations are crucial to solidifying Ketamir-2’s position as a superior alternative to traditional ketamine.
Potential Impact
The pre-clinical results underscore Ketamir-2’s potential as a safer and more effective treatment option for patients suffering from depression and TRD. The oral administration route and favorable safety profile make Ketamir-2 a promising candidate in the field of neuropsychiatric treatment.
Insights
- Stock Surge: MIRA Pharmaceuticals’ stock increased dramatically due to positive pre-clinical trial results.
- Efficacy and Safety: Ketamir-2 shows higher efficacy and better safety than traditional ketamine.
- Behavioral Benefits: Ketamir-2 demonstrated anxiolytic and antidepressant effects in animal tests.
- Patient Compliance: Oral administration of Ketamir-2 promises better compliance compared to traditional ketamine.
- Future Prospects: IND application and human trials are planned by the end of 2024.
The Essence (80/20)
Core Topics:
- Stock Market Reaction: A significant stock price increase following positive trial results.
- Drug Efficacy and Safety: Ketamir-2 shows superior efficacy and safety in pre-clinical trials compared to traditional ketamine.
- Behavioral Test Results: Demonstrated benefits in reducing anxiety and depressive symptoms in animal models.
- Pharmacological Profile: Favorable stability and metabolism suggest potential for human use.
- Oral Administration: Easier administration and better compliance with oral formulation.
- Regulatory Pathway: Plans to file an IND application and commence human clinical trials.
The Action Plan – What MIRA Pharmaceuticals Will Do Next
- Prepare IND Application: Ensure comprehensive data from pre-clinical trials to support the application.
- Engage with FDA: Begin discussions with the FDA to streamline the approval process.
- Continue Research: Conduct additional safety and efficacy studies, focusing on PTSD and neuropathic pain.
- Investor Relations: Maintain clear communication with investors regarding progress and future plans.
- Clinical Trial Preparation: Set up protocols and recruit necessary expertise for human trials.
Blind Spots
Overlooked Detail: The long-term effects and potential risks of high-dose oral Ketamir-2 need thorough investigation. Ensuring robust long-term safety data will be crucial for regulatory approval and market acceptance.
Market Competition: The potential competition from other companies developing similar treatments for neurological and neuropsychiatric disorders. Understanding competitor progress and positioning Ketamir-2 effectively in the market is crucial.
Regulatory Challenges: The possibility of unexpected regulatory hurdles despite promising pre-clinical results. Anticipating and preparing for possible delays or additional requirements from regulatory bodies will be essential for a smooth progression to human trials.
MIRA Technical Analysis
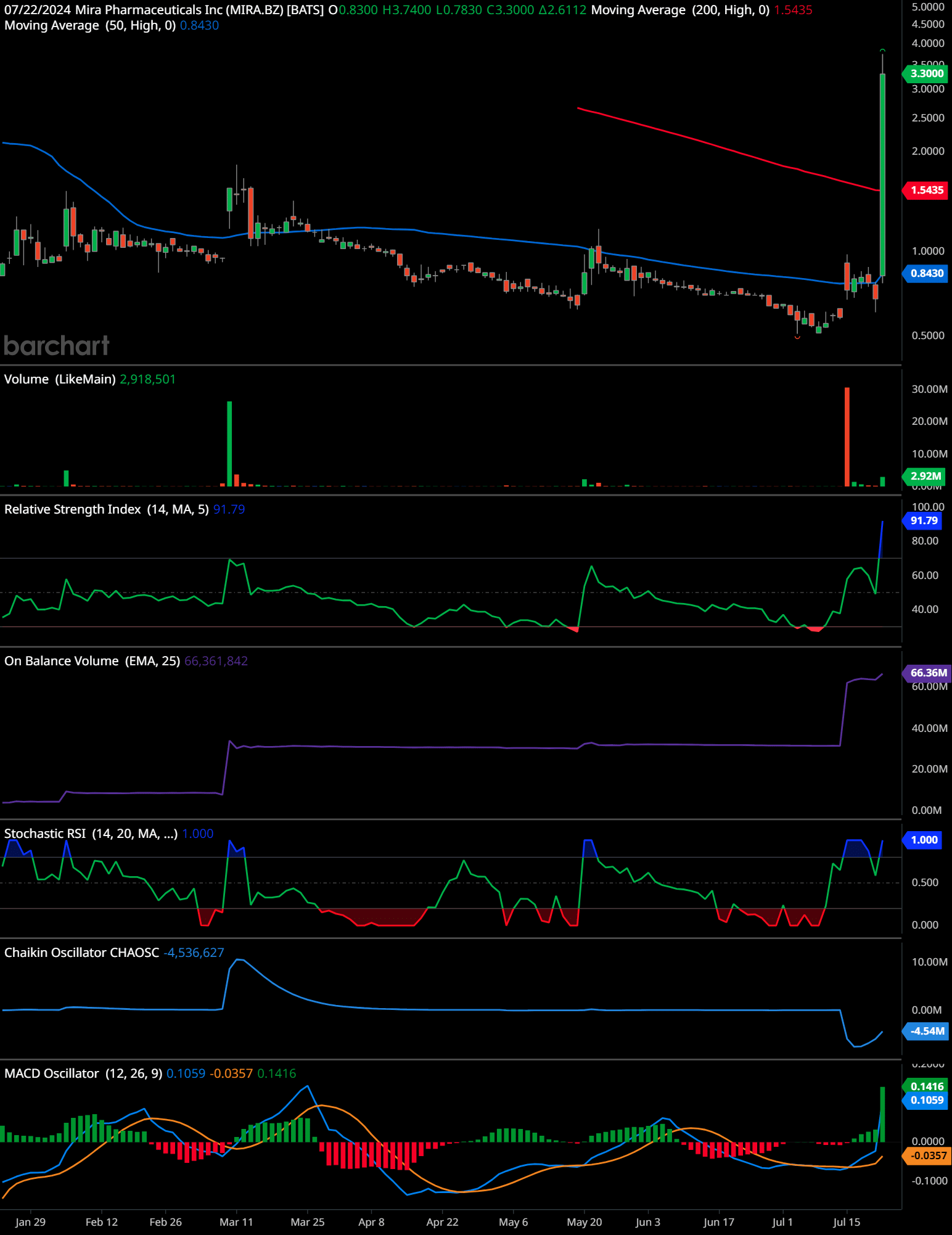
The chart of Mira Pharmaceuticals Inc (MIRA) shows a significant upward price movement with a notable increase in trading volume. Here are the key elements identified in the analysis:
The stock price has recently spiked from around $0.83 to $3.30, indicating a strong bullish movement. The price is currently above both the 50-day moving average (blue line at $0.8430) and the 200-day moving average (red line at $1.5435), which typically suggests a bullish trend.
Volume has increased significantly, with the latest bar showing 2.918 million shares traded. This surge in volume supports the price movement, indicating strong buying interest.
The Relative Strength IndexIn the world of technical analysis, the Relative Strength Index (RSI) stands as a cornerstone tool for traders seeking insights into market momentum. Developed by J. Welles Wilder ... More (RSI) is at 91.79, which is in the overbought territory. This suggests that the stock might be overbought and could be due for a correction or consolidation.
The On-Balance VolumeThe On Balance Volume indicator (OBV) is a technical analysis tool used to measure the flow of money into and out of a security over a specified period of time. It is a cumulative ... More (OBV) is at 66.361 million, indicating that the stock has been accumulating strong volume over time, further supporting the bullish trend.
The Stochastic RSIIn the realm of technical analysis, the Stochastic RSI (StochRSI) emerges as a powerful tool for traders seeking to navigate market dynamics with precision. Developed by Tushar S. ... More is at 1.000, indicating an overbought condition. This aligns with the RSI, suggesting the potential for a pullback.
The Chaikin OscillatorNamed after its creator Marc Chaikin, the Chaikin Oscillator stands as a formidable tool in the arsenal of technical analysts. This oscillator is designed to measure the accumulati... More is at 4.536 million, indicating positive accumulation and distribution, which supports the bullish trend.
The MACDThe MACD indicator is essentially a momentum indicator that shows the relationship between two different moving averages of price. The MACD is the difference between the 12-period ... More Oscillator shows a bullish crossover, with the MACD line (blue) crossing above the signal line (orange) and the histogram moving into positive territory at 0.1416, indicating strong upward momentum.
Time-Frame Signals:
3 Months: Buy – The recent strong upward movement and increased volume suggest continued bullish momentum in the short term. However, the overbought conditions indicated by the RSI and Stochastic RSI should be monitored for potential pullbacks.
6 Months: Hold – While the current trend is bullish, the overbought indicators suggest the possibility of a short-term correction. Holding the stock would be advisable to see how the market consolidates after this significant move.
12 Months: Hold – Given the strong bullish signal but also the potential for correction, it is prudent to hold and monitor the stock for sustained performance or signs of reversal.
Past performance is not an indication of future results, and this analysis should not be considered as investment advice. Always conduct your own research and consider consulting with a financial advisor before making any investment decisions. 🧡
Looking Ahead
MIRA Pharmaceuticals’ breakthrough with Ketamir-2 represents a significant advancement in the treatment of neurological and neuropsychiatric disorders. The positive pre-clinical trial results have not only boosted investor confidence but also paved the way for potential clinical trials that could bring a new, effective, and safer treatment option to patients. As the company moves towards filing an IND application and continues its studies, the future looks promising for Ketamir-2 and MIRA Pharmaceuticals.
FAQs about MIRA Pharmaceuticals and Ketamir-2
1. What caused the significant rise in MIRA Pharmaceuticals’ stock?
The stock rose by 318% following the company’s announcement of positive results from a pre-clinical trial of its novel oral ketamine analog, Ketamir-2.
2. What is Ketamir-2?
Ketamir-2 is a novel oral ketamine analog being developed by MIRA Pharmaceuticals for the treatment of various neurological and neuropsychiatric disorders.
3. What disorders is Ketamir-2 being developed to treat?
Ketamir-2 is being developed to treat depression, treatment-resistant depression (TRD), and post-traumatic stress disorder (PTSD).
4. What are the key findings from the pre-clinical trial of Ketamir-2?
The pre-clinical trial results showed that Ketamir-2 has a higher efficacy and better safety profile compared to traditional oral ketamine, with potential for treating neurological and neuropsychiatric disorders.
5. How does Ketamir-2 compare to traditional oral ketamine?
Ketamir-2 showed higher efficacy and a better safety profile, allowing for tenfold higher doses without major sedative and other side effects observed with traditional ketamine.
6. What behavioral tests were conducted in the pre-clinical trial?
The tests included the Open Field Test (OFT), Elevated Plus Maze (EPM), and Forced Swim Test (FST), all of which demonstrated significant benefits of Ketamir-2.
7. What were the results of the behavioral tests?
Mice treated with Ketamir-2 exhibited reduced anxiety levels, significant anti-depressive effects, and more time spent in open arms in the Elevated Plus Maze test.
8. What advantages does Ketamir-2 offer in terms of administration and compliance?
Ketamir-2 is designed for oral use, unlike traditional ketamine which requires intravenous or intranasal administration, promising better patient compliance and ease of administration.
9. Is Ketamir-2 classified as a controlled substance?
No, Ketamir-2 is not classified as a controlled substance under DEA rules, which could simplify its regulatory pathway.
10. What are the future directions for Ketamir-2?
MIRA Pharmaceuticals aims to file an Investigational New Drug (IND) application with the FDA by the end of 2024 to commence human clinical trials.
11. What ongoing studies are being conducted on Ketamir-2?
Ongoing studies are evaluating Ketamir-2’s efficacy in treating PTSD and neuropathic pain, as well as conducting additional safety studies.
12. Why is Ketamir-2 considered a superior alternative to traditional ketamine?
The pre-clinical results indicate that Ketamir-2 offers a new, safer, and orally effective treatment option for patients suffering from depression and TRD.
💥 GET OUR LATEST CONTENT IN YOUR RSS FEED READER
We are entirely supported by readers like you. Thank you.🧡
This content is provided for informational purposes only and does not constitute financial, investment, tax or legal advice or a recommendation to buy any security or other financial asset. The content is general in nature and does not reflect any individual’s unique personal circumstances. The above content might not be suitable for your particular circumstances. Before making any financial decisions, you should strongly consider seeking advice from your own financial or investment advisor.