On July 30, 2024, Imunon (IMNN) stock surged by 258.5% following the release of positive results from the Phase 2 OVATION 2 Study for their ovarian cancer treatment, IMNN-001. Key findings revealed an 11.1-month increase in overall survival for newly diagnosed advanced ovarian cancer patients and a hazard ratio of 0.74, indicating a 35% improvement in survival. The results, considered clinically significant, generated excitement among investors and the medical community, leading to a trading volume of over 36 million shares, far surpassing the average of 103,000. Imunon plans to advance IMNN-001 into a Phase 3 study and has scheduled an End-of-Phase 2 meeting with the FDA. IMNN-001, an IL-12 immunotherapy, differs from PARP inhibitors and standard chemotherapies in its mechanism of action, showing potential in combination therapies. Further comparisons to existing treatments highlight its promise but note the need for Phase 3 data for definitive conclusions.
Groundbreaking Results from the OVATION 2 Study
Key Findings
The Phase 2 OVATION 2 Study of IMNN-001 in combination with standard chemotherapy yielded groundbreaking results for patients newly diagnosed with advanced ovarian cancer. The study highlighted several critical outcomes:
- Overall Survival Rate: Patients receiving IMNN-001 experienced an 11.1-month increase in overall survival compared to those receiving standard chemotherapy alone.
- Hazard Ratio: The study reported a hazard ratio of 0.74 in the intent-to-treat population, signifying a 35% improvement in survival rates.
Dr. Premal H. Thaker, Interim Chief of Gynecologic Oncology at Washington University School of Medicine and Chair of the OVATION 2 Study, emphasized the significance of these findings. He noted that extending survival from 40 months with standard care to 51 months with IMNN-001 is highly compelling, given that a six-month survival increase is typically considered meaningful in cancer treatment.
Market and Medical Community Reaction
The positive data from the OVATION 2 Study generated considerable excitement within the medical community and among investors. By the morning of July 30, more than 36 million shares of IMNN had been traded, a stark contrast to the daily average trading volume of 103,000 shares. This surge in trading activity reflects the high level of optimism surrounding IMNN-001’s potential to improve outcomes for women with advanced ovarian cancer.
Future Steps for IMNN-001
Phase 3 Pivotal Study
In light of the encouraging results from the Phase 2 trial, Imunon has announced plans to advance IMNN-001 into a Phase 3 pivotal study. The company aims to conduct an End-of-Phase 2 meeting with the U.S. Food and Drug Administration (FDA) to discuss the Phase 3 trial plans, which are expected to commence in the first quarter of 2025.
Investor and Analyst Engagement
To further communicate the promising results and future steps, Imunon has scheduled a conference call with investors and analysts. This call will provide an opportunity for the company to elaborate on the OVATION 2 Study findings and outline the roadmap for advancing IMNN-001 through the next phase of clinical development.
Comparative Analysis of IMNN-001 with Existing Ovarian Cancer Treatments
Efficacy
The Phase 2 results demonstrated that IMNN-001, combined with standard chemotherapy, significantly improved overall survival in newly diagnosed advanced ovarian cancer patients. The 11.1-month increase in survival and the 35% improvement indicated by the hazard ratio of 0.74 are notable achievements. In comparison, PARP inhibitors such as olaparib and niraparib have shown hazard ratios of 0.29 and 0.25, respectively, for progression-free survival in BRCA-mutated ovarian cancer.
Mechanism of Action
IMNN-001 is an interleukin-12 (IL-12) immunotherapy based on TheraPlas™ technology, which differs from PARP inhibitors that target DNA repair mechanisms or standard chemotherapies like carboplatin that directly damage cancer cells.
Combination Potential
The study revealed that IMNN-001, when combined with standard chemotherapy and PARP inhibitors, showed promising results. The hazard ratio of 0.41 for overall survival in this subgroup suggests that IMNN-001 may enhance the efficacy of existing treatments.
Stage of Development
While IMNN-001 is still in Phase 2 trials, PARP inhibitors and platinum-based chemotherapies are already approved as standards of care. This places IMNN-001 at a critical juncture, where the forthcoming Phase 3 trial will be crucial in determining its potential to become a new standard treatment.
Patient Population
IMNN-001 is being studied in newly diagnosed advanced ovarian cancer patients. PARP inhibitors, on the other hand, have shown particular efficacy in BRCA-mutated and homologous recombination deficiency (HRD)-positive ovarian cancers.
Administration
IMNN-001 is administered intraperitoneally in combination with chemotherapy, differing from oral PARP inhibitors or intravenous chemotherapies.
Insights:
- IMNN-001 increased overall survival by 11.1 months for advanced ovarian cancer patients.
- The 258.5% stock surge reflects strong investor confidence.
- Imunon is preparing for a Phase 3 trial and discussions with the FDA.
- IMNN-001 shows potential in combination with other cancer treatments.
The Essence (80/20)The Origins and Evolution of the 80/20 Principle The Discovery by Vilfredo Pareto In 1897, Italian economist Vilfredo Pareto uncovered a striking pattern in his study of wealth and... More: The core of this development lies in the significant survival benefits observed with IMNN-001 in advanced ovarian cancer patients. The 11.1-month increase in overall survival and a 35% improvement indicated by the hazard ratio underscore its potential as a new standard of care. The anticipation for Phase 3 trials and regulatory discussions highlights the forward momentum of IMNN-001. Its unique mechanism as an IL-12 immunotherapy, and promising combination potential with existing treatments, set it apart from current options like PARP inhibitors and standard chemotherapies.
The Action Plan – What Imunon Should Do Next:
- Advance to Phase 3 Trials: Expedite the commencement of Phase 3 trials to validate the efficacy and safety of IMNN-001.
- Regulatory Engagement: Conduct an End-of-Phase 2 meeting with the FDA to ensure regulatory alignment and address any potential concerns.
- Stakeholder Communication: Maintain transparent communication with investors and the medical community through updates and conference calls.
- Combination Studies: Continue exploring combination therapies with IMNN-001 to enhance treatment outcomes.
Blind Spot: While the results are promising, the comparison with other treatments remains indirect due to differences in study designs and patient populations. The long-term safety and broader efficacy of IMNN-001 need more comprehensive data from Phase 3 trials.
IMNN Technical Analysis Daily Time Frame
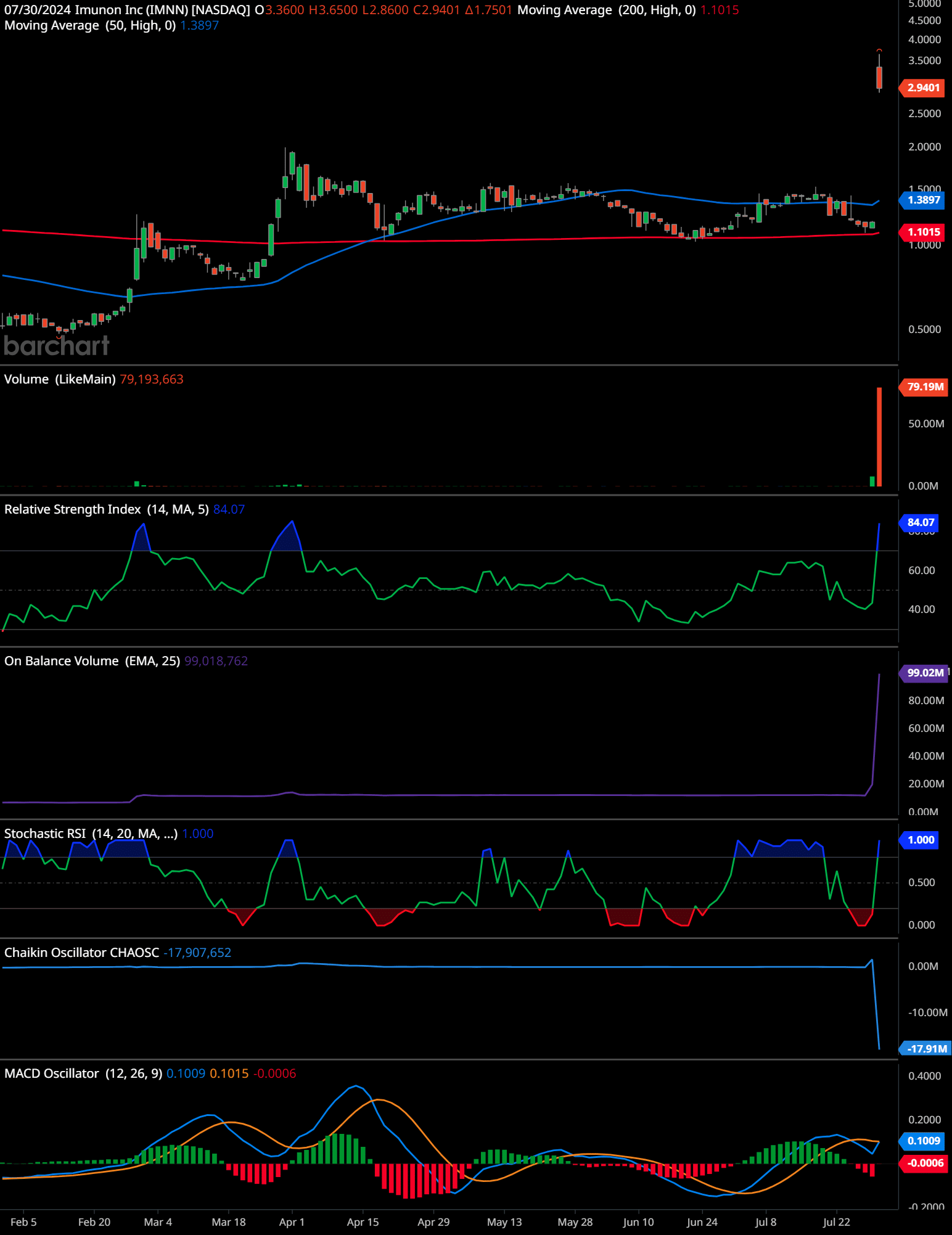
Based on the provided chart for Imunon (IMNN), here is a comprehensive technical analysis:
Trend Analysis:
The stock experienced a significant spike in late February, reaching a high of approximately 3.65 before declining and stabilizing around 1.30. Recently, there’s another sharp rise in price, closing at 2.94.
Support and Resistance Levels:
Key resistance level is around 3.65, the high from late February.
Key support level is around 1.10, observed multiple times as the lowest point during consolidation phases.
Volume:
There’s a substantial increase in trading volume, particularly on the last trading day, indicating heightened investor interest and possible continuation of the price movement.
Relative Strength IndexIn the world of technical analysis, the Relative Strength Index (RSI) stands as a cornerstone tool for traders seeking insights into market momentum. Developed by J. Welles Wilder ... More (RSI):
The RSI is at 84.07, which is in the overbought territory. This suggests that the stock might be overvalued in the short term and could be due for a correction.
On Balance VolumeThe On Balance Volume indicator (OBV) is a technical analysis tool used to measure the flow of money into and out of a security over a specified period of time. It is a cumulative ... More (OBV):
The OBV shows a significant upward movement, which supports the recent price increase and suggests that the upward momentum might continue.
Stochastic RSIIn the realm of technical analysis, the Stochastic RSI (StochRSI) emerges as a powerful tool for traders seeking to navigate market dynamics with precision. Developed by Tushar S. ... More:
The Stochastic RSI is at 1.000, indicating that the stock is currently overbought. Similar to the RSI, this signals potential for a short-term pullback.
Chaikin OscillatorNamed after its creator Marc Chaikin, the Chaikin Oscillator stands as a formidable tool in the arsenal of technical analysts. This oscillator is designed to measure the accumulati... More:
The Chaikin Oscillator is at -17,907,652, suggesting recent bearish accumulation or distribution, which could be a warning sign despite the price rise.
MACDThe MACD indicator is essentially a momentum indicator that shows the relationship between two different moving averages of price. The MACD is the difference between the 12-period ... More:
The MACD is showing a crossover above the signal line, which is a bullish indicator. However, the histogram’s recent movement suggests that this bullish momentum might be weakening.
Time-Frame Signals:
3 Months:
Signal: Hold
The stock has shown a strong upward movement but is currently overbought according to multiple indicators. It is prudent to wait and see if this momentum sustains or if a correction occurs.
6 Months:
Signal: Buy
If the stock can maintain above the support level of 1.10 and potentially break past the resistance level of 3.65, it indicates strong bullish momentum that can be capitalized on over a medium-term horizon.
12 Months:
Signal: Hold
Given the volatility and sharp movements observed, it is important to see how the stock performs in the medium term. Maintaining a hold recommendation allows for reassessment based on emerging patterns and fundamental developments.
This analysis takes into account the current technical indicators and trends, providing a comprehensive view for different investment horizons.
IMNN Technical Analysis Weekly Time Frame
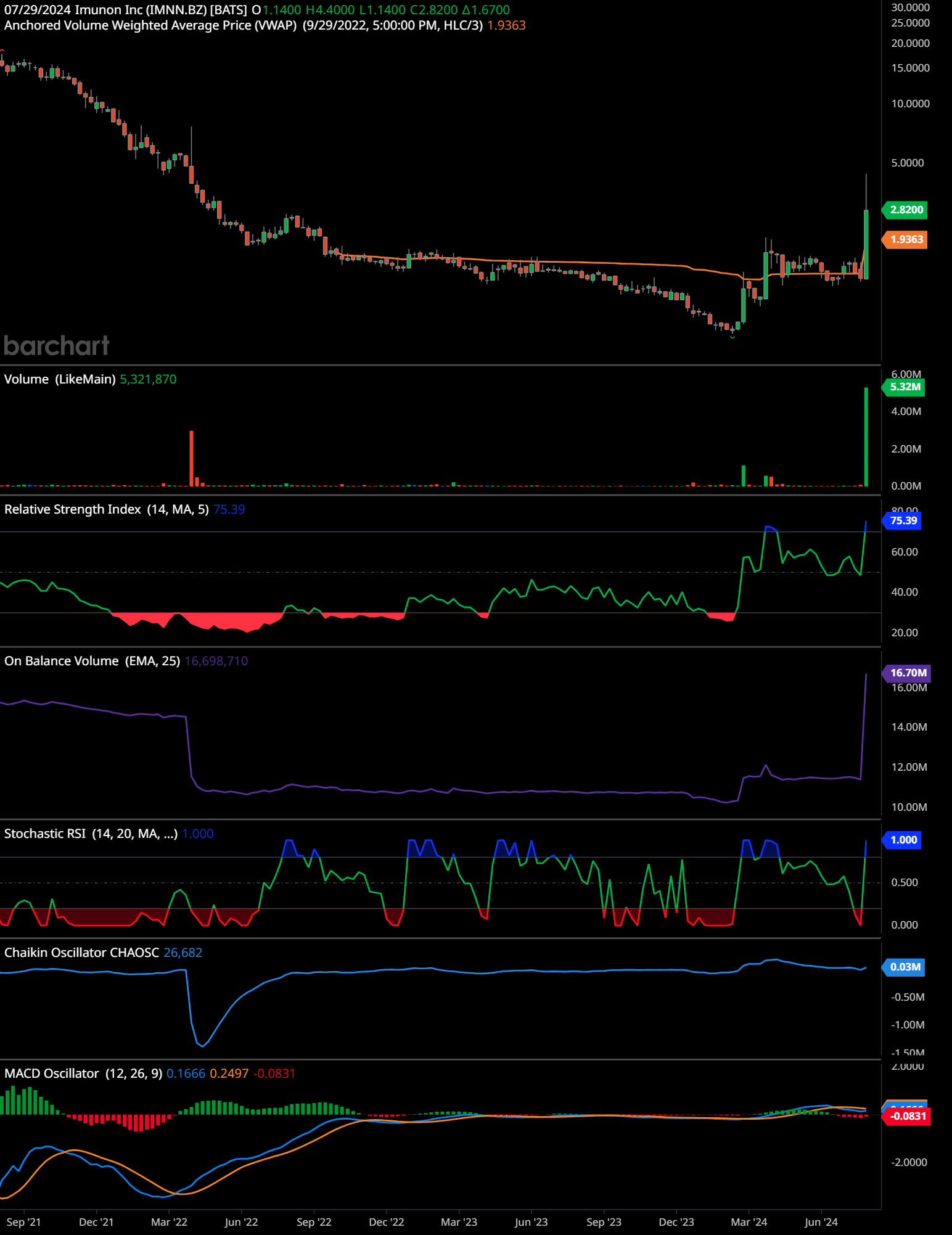
In the weekly time frame, Immuno Inc (IMNN) shows a significant shift in momentum. The price had been in a prolonged downward trend, reaching lows around 1.1400. However, recent weeks exhibit a sharp increase, closing at 2.8200, indicating a potential reversal.
Volume analysis reveals a substantial spike, suggesting heightened trading activity and interest. The Relative Strength Index (RSI) has moved up to 75.39, which is above the overbought threshold of 70, potentially signaling that the stock might be overbought.
The On-Balance Volume (OBV) indicator has seen a notable increase, confirming that volume is flowing into the stock. The Stochastic RSI has hit 1.000, also indicating overbought conditions, while the Chaikin Oscillator shows a value of 26.682, suggesting accumulation.
The MACD oscillator’s recent crossover into positive territory at 0.1666 indicates bullish momentum, but the histogram’s slight decline to -0.0831 warrants caution as it might suggest weakening momentum.
Time-Frame Signals:
3 months: Hold
6 months: Hold
12 months: Buy
Past performance is not an indication of future results. This article should not be considered as investment advice. Always conduct your own research and consider consulting with a financial advisor before making any investment decisions 🧡.
The Road Ahead for IMNN-001
While IMNN-001 shows promising results, particularly when used in combination with other therapies, it is essential to recognize that direct comparisons with existing treatments are limited due to differences in study designs, patient populations, and stages of clinical development. The ongoing Phase 3 trial will provide more definitive data on how IMNN-001 compares to current standard treatments and its potential to redefine the standard of care for advanced ovarian cancer. As Imunon progresses with its clinical development, the medical community and investors alike will be closely monitoring the outcomes, hopeful that IMNN-001 will offer a significant breakthrough in ovarian cancer treatment.
Imunon (IMNN) Stock Surge FAQ
Frequently Asked Questions
- 1. Why did Imunon (IMNN) stock surge by 258.5% on July 30, 2024?
- Imunon stock surged due to the release of highly positive results from a Phase 2 clinical trial for their advanced ovarian cancer treatment, IMNN-001.
- 2. What were the key findings from the Phase 2 OVATION 2 Study?
- The key findings include an 11.1-month increase in overall survival rate for newly diagnosed patients with advanced ovarian cancer and a hazard ratio of 0.74, indicating a 35% improvement in survival.
- 3. How significant are these clinical trial results?
- These results are considered clinically significant because a six-month survival increase is typically regarded as meaningful in cancer treatment. The extension of survival from 40 months to 51 months with IMNN-001 is particularly compelling.
- 4. Who is Dr. Premal H. Thaker?
- Dr. Premal H. Thaker is the Interim Chief of Gynecologic Oncology at Washington University School of Medicine and Chair of the OVATION 2 Study.
- 5. How did the positive data affect the trading of IMNN shares?
- The positive data generated excitement in the medical community and among investors, leading to heavy trading of IMNN shares, with more than 36 million shares changing hands compared to the daily average of 103,000 shares.
- 6. What are Imunon’s plans following the Phase 2 trial results?
- Imunon plans to advance IMNN-001 into a Phase 3 pivotal study, conduct an End-of-Phase 2 meeting with the FDA to discuss plans for the Phase 3 trial, and commence the trial in the first quarter of 2025.
- 7. How does IMNN-001 compare to other ovarian cancer treatments in terms of efficacy?
- IMNN-001 showed an 11.1-month increase in overall survival with a hazard ratio of 0.74, indicating a 35% improvement. In comparison, PARP inhibitors like olaparib and niraparib have hazard ratios of 0.29 and 0.25 for progression-free survival in BRCA-mutated ovarian cancer.
- 8. What is the mechanism of action of IMNN-001?
- IMNN-001 is an interleukin-12 (IL-12) immunotherapy based on TheraPlas™ technology, differing from PARP inhibitors and standard chemotherapies that target DNA repair mechanisms or directly damage cancer cells.
- 9. Can IMNN-001 be combined with other treatments?
- Yes, IMNN-001 showed promising results when combined with standard chemotherapy and PARP inhibitors, with a hazard ratio of 0.41 for overall survival in this subgroup, suggesting it may enhance the efficacy of existing treatments.
- 10. What are the next steps for IMNN-001’s development?
- The next steps include advancing IMNN-001 into a Phase 3 pivotal study, holding an End-of-Phase 2 meeting with the FDA, and starting the Phase 3 trial in the first quarter of 2025.
💥 GET OUR LATEST CONTENT IN YOUR RSS FEED READER
We are entirely supported by readers like you. Thank you.🧡
This content is provided for informational purposes only and does not constitute financial, investment, tax or legal advice or a recommendation to buy any security or other financial asset. The content is general in nature and does not reflect any individual’s unique personal circumstances. The above content might not be suitable for your particular circumstances. Before making any financial decisions, you should strongly consider seeking advice from your own financial or investment advisor.