Sarepta Therapeutics’ stock is rising due to its inclusion in the S&P MidCap 400, expanded FDA approval for its gene therapy Elevidys, and the failure of Pfizer’s competing therapy. The FDA’s approval now covers about 80% of Duchenne muscular dystrophy (DMD) patients, significantly broadening the market. Despite previous skepticism and conditional approval issues, Elevidys has shown promising results in producing the protein microdystrophin, offering hope for stabilizing DMD. The gene therapy market is expected to grow substantially, driven by technological advancements and increased funding, though challenges like high costs and complex administration remain.
Inclusion in the S&P MidCap 400
On June 3, 2024, Sarepta Therapeutics was added to the S&P MidCap 400 index. This inclusion is a significant milestone for the company, as it brings increased visibility and attracts investment from index funds and institutional investors. Historically, such inclusions lead to a rise in stock prices due to the heightened demand from these investment entities. For Sarepta, this has been a crucial driver in the recent uptick in its stock price.
Positive Regulatory Developments for Elevidys
Expanded FDA Approval
On June 20, 2024, the FDA granted expanded approval for Sarepta’s gene therapy, Elevidys. Originally approved in June 2023 for a limited group of boys aged 4 to 5 years with Duchenne muscular dystrophy (DMD), the new approval significantly broadens its market. Elevidys is now available for all DMD patients aged four and above, regardless of their ability to walk. This expansion potentially covers about 80% of Duchenne patients, a significant increase from the initial 400 boys per year.
Full Approval for Ambulatory Patients
For those still able to walk, the FDA converted Elevidys’ conditional approval to full approval. This means the therapy’s market availability for ambulatory patients is no longer contingent on additional tests. For non-ambulatory patients, however, the clearance is conditional on the results of the ongoing Phase 3 Envision study. This regulatory development is a major boost for Sarepta, enhancing its market presence and driving investor optimism.
Competitive Landscape: Pfizer’s Trial Failure
Another significant factor contributing to Sarepta’s stock surge is the failure of a competitor’s trial. Pfizer’s gene therapy for DMD, fodadistrogene movaparvovec, failed to meet its primary and secondary endpoints in a Phase 3 trial. This failure reduces competitive pressure on Sarepta’s Elevidys, potentially allowing it to capture a larger share of the market without immediate competition. This scenario has further bolstered investor confidence in Sarepta’s prospects.
The Broader Impact of Elevidys’ Approval
Addressing Unmet Needs
Duchenne muscular dystrophy is a progressive and fatal muscle-wasting condition that primarily affects boys. Patients gradually lose their ability to walk, typically during their teenage years, and often die from heart or lung complications in their 30s. Existing treatments, like steroids and exon-skipping drugs, offer only modest benefits and come with significant side effects.
Elevidys aims to address this urgent treatment need by helping the body produce a miniature form of the protein dystrophin, which is lacking in DMD patients. The expanded approval of Elevidys represents a significant advancement in the treatment of this devastating disease, potentially stabilizing its progression.
Market Potential and Financial Implications
With a list price of $3.2 million, Elevidys is one of the world’s most expensive medicines. The broader approval could accelerate sales, which have totaled a cumulative $334 million but appeared to flatten recently. The expanded market coverage could lead to a substantial increase in revenue for Sarepta, enhancing its financial outlook.
Challenges and Considerations
Effectiveness and Safety Concerns
Despite its expanded approval, questions remain about Elevidys’ effectiveness. In a Phase 2 study, the therapy did not show a meaningful difference in motor function compared to a placebo. However, some advocates and researchers argue that the therapy has shown stabilization of the disease, which is a significant achievement.
Safety is another concern. While most side effects are manageable with monitoring and immune-suppressing drugs, there have been instances of serious liver damage and muscle weakness. Moreover, receiving Elevidys may preclude patients from getting different gene therapies in the future.
Regulatory Scrutiny
The FDA’s decision to expand Elevidys’ approval has not been without controversy. The initial conditional approval was based on the production of microdystrophin, deemed “reasonably likely” to predict a benefit. If a confirmatory study fails, the therapy’s approval could be withdrawn. This regulatory scrutiny adds an element of uncertainty to Sarepta’s prospects.
Future Outlook for the Gene Therapy Market
Projected Growth
The gene therapy market is poised for substantial growth over the next decade. The global market, valued at approximately $8.75 billion in 2023, is expected to reach around $52.40 billion by 2033, growing at a CAGR of 19.6%. The U.S. market, estimated at $1.59 billion in 2023, is projected to surpass $9.44 billion by 2033, with a CAGR of 19.50%.
Regional Insights
North America dominated the market in 2023, with a revenue share of 65.12%. Europe is expected to be the fastest-growing region from 2024 to 2030, driven by a large population with unmet medical needs. The Asia Pacific region is also anticipated to witness significant growth due to increased government investments and local presence of major companies.
Market Drivers and Challenges
Technological advancements in gene editing, increased funding, and regulatory approvals are key drivers of market growth. However, high costs and complex administration pose significant challenges. Gene therapies are expensive, often requiring extended hospital stays and supplementary services, adding to the overall cost and complexity.
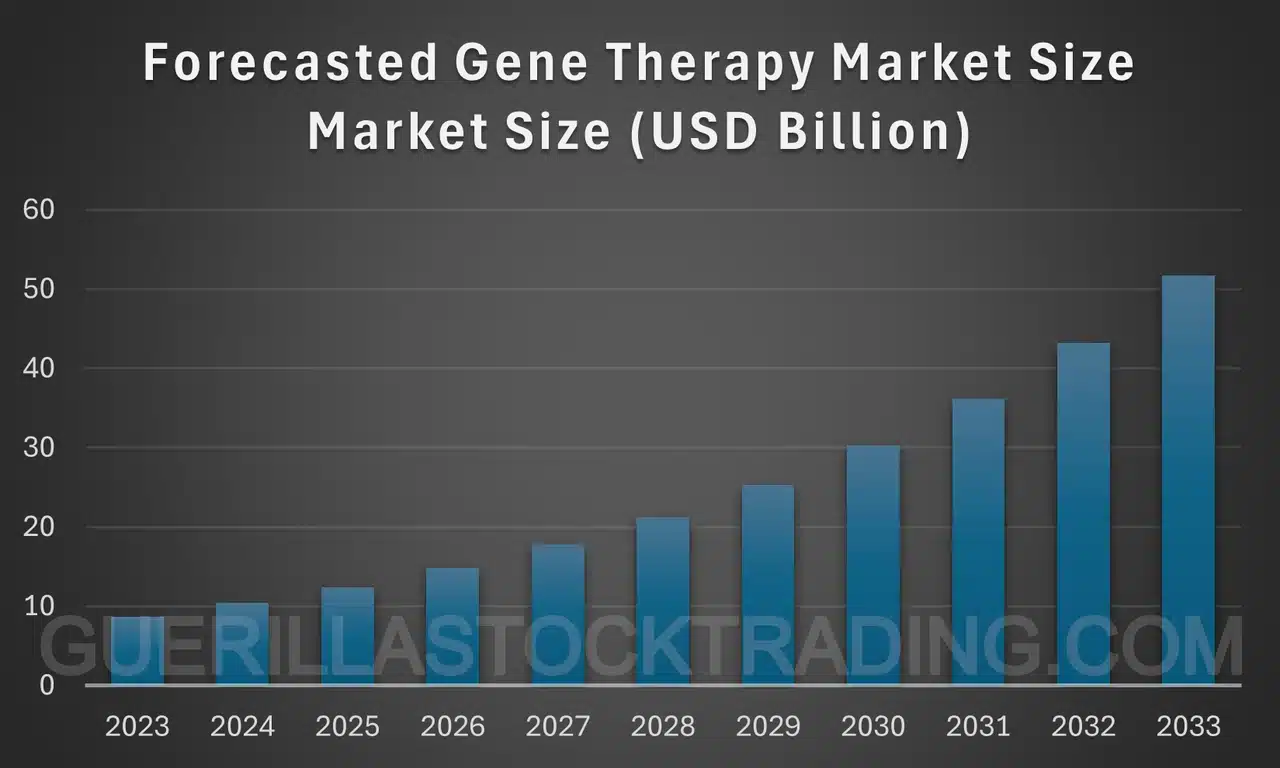
The gene therapy market is projected to experience significant growth over the next decade. Here are the key forecasts and insights:
Global Market
- Market Size in 2023: The global gene therapy market was valued at approximately USD 8.75 billion.
- Projected Market Size by 2033: It is expected to reach around USD 52.40 billion by 2033.
- Compound Annual Growth RateThe world of finance is replete with complex concepts, but one that stands as a cornerstone for investors seeking to gauge returns is the Compound Annual Growth Rate (CAGR). Often ... More (CAGR): The market is anticipated to grow at a CAGR of 19.6% from 2024 to 2033[1].
U.S. Market
- Market Size in 2023: The U.S. gene therapy market was estimated at USD 1.59 billion.
- Projected Market Size by 2033: It is expected to surpass USD 9.44 billion by 2033, growing at a CAGR of 19.50% from 2024 to 2033[1].
- Alternative Estimate: Another source estimates the U.S. market size at USD 3.19 billion in 2023, with a projection to grow to USD 18.50 billion by 2033 at a CAGR of 19.22%[2].
Regional Insights
- North America: Dominated the market in 2023 with a revenue share of 65.12%. The region is expected to continue leading in terms of approvals and revenue generation due to significant investments in R&D and the prevalence of targeted diseases[1].
- Europe: Expected to be the fastest-growing regional segment from 2024 to 2030, driven by a large population with unmet medical needs and increasing demand for novel technologies[1].
- Asia Pacific: Anticipated to witness significant growth due to the availability of resources, local presence of major companies, and increased government investments[1].
Market Drivers
- Technological Advancements: Innovations in gene editing (e.g., CRISPR-Cas9), viral vector development, and delivery mechanisms have propelled the market from speculation to clinical application[2].
- Increased Funding and Investments: Significant investments in gene therapy R&D and manufacturing capabilities are expected to provide lucrative growth opportunities. For instance, Ori Biotech raised over USD 100 million in 2022 for a novel cell and gene therapy platform[2].
- Regulatory Approvals: As of March 2024, there are 36 gene therapies approved by the FDA, with an additional 500 in the pipeline. It is expected that 10-20 new gene therapies will be approved annually by 2025[4].
Challenges
- High Costs: Gene therapies are expensive, with some treatments costing millions of dollars per patient. This poses financial challenges for healthcare systems and plan sponsors[4].
- Complex Administration: The administration of gene therapies often involves extended hospital stays and supplementary services, adding to the overall cost and complexity[4].
In summary, the gene therapy market is poised for robust growth driven by technological advancements, increased funding, and a growing pipeline of therapies. However, the high costs and complex administration of these therapies remain significant challenges.
Insights
- Sarepta’s inclusion in S&P MidCap 400 boosts investor visibility.
- FDA’s expanded approval increases Elevidys’ market significantly.
- Failure of Pfizer’s therapy reduces competition for Sarepta.
- Gene therapy market projected to grow rapidly, with significant investments.
The Essence (80/20)The Origins and Evolution of the 80/20 Principle The Discovery by Vilfredo Pareto In 1897, Italian economist Vilfredo Pareto uncovered a striking pattern in his study of wealth and... More
- Core Topics:
- Inclusion in S&P MidCap 400: Boosts visibility and investment.
- FDA Approval: Expanded coverage for Elevidys, covering 80% of DMD patients.
- Competitive Landscape: Pfizer’s trial failure benefits Sarepta.
- Market Growth: Gene therapy market expected to grow significantly due to advancements and funding.
The Guerilla Stock Trading Action Plan
- Monitor Stock: Track Sarepta’s stock performance post-inclusion.
- Regulatory Updates: Stay updated on further FDA decisions regarding Elevidys.
- Market Analysis: Evaluate the impact of reduced competition on Sarepta’s market share.
- Investment Opportunities: Consider investing in the growing gene therapy market, focusing on companies with promising advancements and approvals.
Blind Spots
- Long-term Efficacy and Safety: Continued scrutiny on Elevidys’ long-term effectiveness and potential side effects could impact its market stability and acceptance.
- Market Access and Affordability: The high cost of Elevidys ($3.2 million per treatment) could limit accessibility for many patients and strain healthcare systems, potentially leading to pushback from insurers and affecting overall market penetration and adoption rates. This financial barrier could also influence public perception and regulatory scrutiny.
Looking Ahead
Sarepta Therapeutics’ recent stock surge is a testament to the positive developments surrounding its gene therapy, Elevidys. The inclusion in the S&P MidCap 400, expanded FDA approval, and reduced competition have created a favorable environment for the company. As the gene therapy market continues to grow, Sarepta is well-positioned to capitalize on these opportunities. However, challenges related to effectiveness, safety, and regulatory scrutiny remain, underscoring the need for continued innovation and vigilance in this promising but complex field.
Sarepta Therapeutics FAQ
Frequently Asked Questions
- What caused Sarepta Therapeutics’ recent stock rise?
- Sarepta Therapeutics’ stock rise is attributed to its inclusion in the S&P MidCap 400, positive regulatory developments for its gene therapy Elevidys, and the failure of Pfizer’s competing gene therapy trial.
- When was Sarepta Therapeutics added to the S&P MidCap 400?
- Sarepta Therapeutics was added to the S&P MidCap 400 index before the market opened on June 3, 2024.
- How did the inclusion in the S&P MidCap 400 affect Sarepta’s stock?
- The inclusion increased visibility and investment from index funds and institutional investors, contributing to the stock price rise.
- What is Elevidys?
- Elevidys is a gene therapy developed by Sarepta Therapeutics for treating Duchenne muscular dystrophy (DMD).
- What recent regulatory development occurred for Elevidys?
- On June 20, 2024, the FDA expanded the approval of Elevidys, significantly increasing its market reach.
- How did the failure of Pfizer’s gene therapy trial impact Sarepta?
- Pfizer’s gene therapy for DMD failed to meet its primary and secondary endpoints, reducing competitive pressure on Sarepta’s Elevidys.
- What age group is Elevidys approved for?
- Elevidys is approved for individuals with Duchenne who are at least four years of age and have mutations in a specific gene.
- What are the conditions for Elevidys’ full approval?
- Elevidys received full approval for ambulatory patients, while non-ambulatory patient approval is contingent on results from the ongoing Phase 3 Envision study.
- What percentage of Duchenne patients are now eligible for Elevidys?
- The new approval covers about 80% of Duchenne patients.
- What is the estimated market size for Elevidys in the U.S.?
- Sarepta estimates the initial approval covered about 400 boys in the U.S. each year, but the expanded approval significantly increases this number.
- What is the list price of Elevidys?
- Elevidys has a list price of $3.2 million, making it one of the world’s most expensive medicines.
- What is Duchenne muscular dystrophy?
- Duchenne muscular dystrophy (DMD) is a progressive and fatal muscle-wasting condition primarily affecting boys, leading to loss of walking ability and complications in the 30s.
- Are there any side effects associated with Elevidys?
- Side effects can include liver damage and muscle weakness, but most are manageable with monitoring and immune-suppressing drugs.
- How does Elevidys work?
- Elevidys helps the body produce microdystrophin, a miniature form of the protein dystrophin, which is lacking in people with Duchenne.
- What are the market projections for gene therapy?
- The global gene therapy market is expected to grow from approximately USD 8.75 billion in 2023 to around USD 52.40 billion by 2033, at a CAGR of 19.6%.
- What factors are driving the growth of the gene therapy market?
- Technological advancements, increased funding, and regulatory approvals are key drivers of the gene therapy market growth.
- What challenges does the gene therapy market face?
- High costs and complex administration are significant challenges in the gene therapy market.
- How does Elevidys compare to Pfizer’s gene therapy?
- Elevidys has not been slowed by safety concerns and shows apparent benefit on multiple secondary measures, differentiating it from Pfizer’s therapy.
- What is the significance of the FDA’s expanded approval for Elevidys?
- The expanded approval allows a broader spectrum of Duchenne patients to access the therapy, addressing an urgent treatment need.
- What is the current status of gene therapies from other companies?
- Other gene therapies from companies like Regenxbio and Solid are years away from possible approval.
Book Recommendations:
“The Future of Gene Therapy: From Cutting-Edge Science to Breakthrough Cures” by Aaron D. Wiegand:
- Relation to Article: This book delves into the scientific advancements and potential of gene therapy, similar to how Elevidys represents a significant breakthrough for treating Duchenne muscular dystrophy (DMD). Understanding the broader context of gene therapy can provide deeper insights into the technological and therapeutic milestones discussed in the article.
“Biotechnology and Biopharmaceuticals: Transforming Proteins and Genes into Drugs” by Rodney J. Y. Ho:
- Relation to Article: The article highlights the development and approval of Sarepta’s Elevidys, a gene therapy for DMD. This book provides a comprehensive overview of the biotechnology industry, focusing on the processes of turning scientific discoveries into therapeutic drugs. It offers a detailed backdrop to the commercial and scientific journey of treatments like Elevidys.
“Genentech: The Beginnings of Biotech” by Sally Smith Hughes:
- Relation to Article: The narrative of Genentech’s rise mirrors Sarepta’s current trajectory in pioneering gene therapies. By understanding the historical context and challenges faced by early biotech companies like Genentech, readers can gain perspective on Sarepta’s achievements and the broader evolution of the biotechnology industry, which is critical for appreciating the significance of developments like those described in the article.
These books provide a deeper understanding of the scientific, commercial, and historical aspects of biotechnology and gene therapy, enriching the reader’s comprehension of the key themes and developments presented in the article.
Citations:
[1] https://www.novaoneadvisor.com/report/gene-therapy-market
[2] https://www.biospace.com/article/releases/u-s-gene-therapy-market-size-share-and-growth-report-2033/
[3] https://www.novaoneadvisor.com/report/cell-and-gene-therapy-market
[4] https://www.segalco.com/consulting-insights/spotlight-on-gene-therapies-in-q2-2024-trends
💥 GET OUR LATEST CONTENT IN YOUR RSS FEED READER
We are entirely supported by readers like you. Thank you.🧡
This content is provided for informational purposes only and does not constitute financial, investment, tax or legal advice or a recommendation to buy any security or other financial asset. The content is general in nature and does not reflect any individual’s unique personal circumstances. The above content might not be suitable for your particular circumstances. Before making any financial decisions, you should strongly consider seeking advice from your own financial or investment advisor.